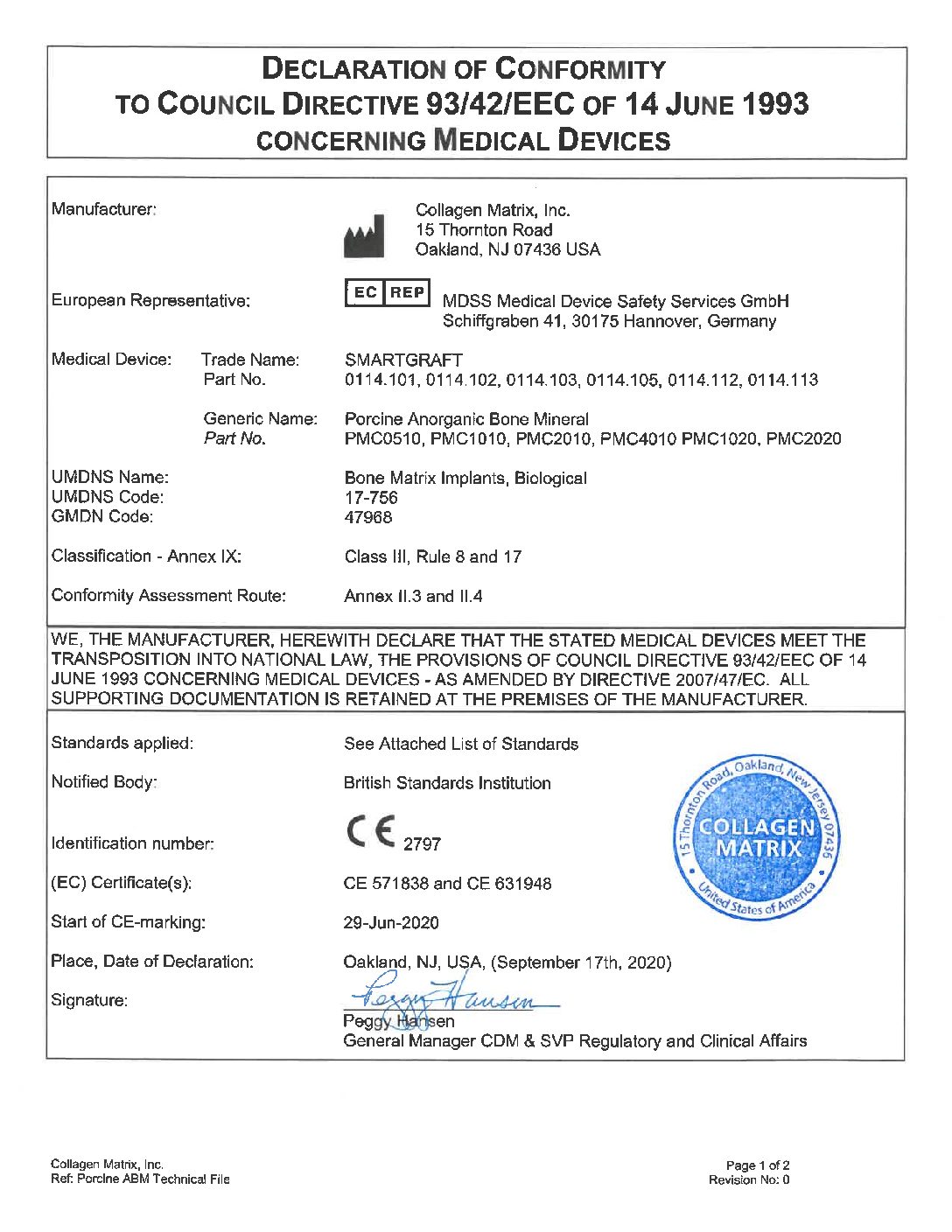
Smartgraft 1cc / 1-2mm
1 Dappen di xenograft suino
Prezzo totale:
Offriamo la spedizione gratuita in tutta Italia per ordini superiori a EUR 1000.
Descrizione
PERCHÉ UTILIZZARE L’INNESTO OSSEO SUINO SMARTGRAFT?
SmartGRAFT è un sostituto osseo che bilancia l’alta porosità con un rimodellamento stabile del volume [1,2]. L’alta porosità e i macro pori migliorano la vascolarizzazione, la crescita dell’osso e l’osteointegrazione dell’impianto dopo l’intervento [3,4].

Adesione cellulare
La superficie ruvida dei granuli di origine suina, simili a quella dell’osso umano, facilita l’adesione di nuove cellule [1,2].
Migrazione / proliferazione
L’alta porosità di SmartGRAFT e i grandi pori migliorano la vascolarizzazione, la crescita dell’osso e l’osteointegrazione dell’impianto dopo l’intervento. La matrice minerale ossea anorganica ha interconnessioni che riducono la densità di massa dell’innesto, assicurando più spazio per la formazione di nuovo tessuto osseo [10]. I macropori di SmartGRAFT vanno da 0,1 mm a 1,0 mm.
L’apatite nativa, conservata dal processo di purificazione brevettato, possiede la sua struttura porosa naturale per la proliferazione cellulare e osteoconduzione.
 RIGENERAZIONE
RIGENERAZIONE
Il sostituto osseo di origine suina presenta una struttura simile all’osso umano favorendo un rimodellamento equilibrato [5].
SEI RAGIONI PER AGGIUNGERE HYADENT BG A SMARTGRAFT
- Il putty può essere preparato in 3 minuti con xHyA Hyadent BG – gel pronto all‘uso – e Smartgraft.
- Come agente idrofilo, l‘Acido Ialuronico (HA) stabilizza il coagulo di sangue e attrae fattori di crescita per sostenere e accelerare la formazione ossea.20-23
- L’acido ialuronico favorisce l’angiogenesi.24
- L‘alto peso molecolare dell‘Acido Ialuronico riduce il gonfiore e il fastidio, supportando la guarigione senza esiti cicatriziali.25
- L’Acido Ialuronico ha proprietà batteriostatiche naturali.26
- La speciale formulazione dell’Acido Ialuronico rimane presente durante le varie fasi del processo di guarigione grazie al suo lento riassorbimento (diverse settimane).22
Letteratura scientifica di Smartgraft
- Deligianni DD, Katsala ND, Koutsoukos PG, Missirlis YF, Effect of Surface Roughness of Hydroxyapatite on Human Bone Marrow Cell Adhesion, Proliferation, Differentiation and Detachment Strength. Elsevier Biomaterials 22 (2001) 87–96
- Shu-Thung L et al. (2014) Isolation and Characterization of a Porous Carbonate Apatite From Porcine Cancellous Bone. Science, Technology, Innovation, Aug: 1-13 (data on file)
- Frank M. Klenke, Yuelian Liu, Huipin Yuan, Ernst B. Hunziker, Klaus A. Siebenrock, Willy Hofstetter. Impact of Pore Size on the Vascularization and Osseointegration of Ceramic Bone Substitutes in vivo. Journal of Biomedical Materials Research Part A, 2007, 777-786
- Hannink G1, Arts JJ. Bioresorbability, porosity and mechanical strength of bone substitutes: what is optimal for bone regeneration? Injury. 2011 Sep;42 Suppl 2:S22-5
- Saghiri MA, Asatourian A, Garcia-Godoy F, Sheibani N. The role of angiogenesis in implant dentistry part II: The effect of bone-grafting and barrier membrane materials on angiogenesis. Med Oral Patol Oral Cir Bucal. 2016 Jul 1;21(4):e526-37. doi: 10.4317/medoral.21200. PMID: 27031074; PMCID: PMC4920468.
- Data on file
- Data on file
- Shu-Thung L et al. (2014) Isolation and Characterization of a Porous Carbonate Apatite From Porcine Cancellous Bone. Science, Technology, Innovation, Aug: 1-13 (data on file)
- Bracey DN, Seyler TM, Jinnah AH, Lively MO, Willey JS, Smith TL, et al. A decellularized porcine xenograft-derived bone scaffold for clinical use as a bone graft substitute: a critical evaluation of processing and structure. J Funct Biomater. 2018;9(3):45.https://doi.org/10.3390/jfb9030045.
- Lai VJ, Michalek JE, Liu Q, Mealey BL. Ridge preservation following tooth extraction using bovine xenograft compared with porcine xenograft: A randomized controlled clinical trial. J Periodontol. 2020 Mar;91(3):361-368. doi: 10.1002/JPER.19-0211. Epub 2019 Aug 23. PMID: 31380563.
- Renzo et al.: Tissue Dimensional Changes Following Alveolar Ridge Preservation with Different Xenografts Associated with a Collagen Membrane. Results at the 4-Month Re-Entry Surgery. Int Arch Oral Maxillofac Surg, 2017, 1:003
- Guarnieri R, Di Nardo D, Di Giorgio G, Miccoli G, Testarelli L. Effectiveness of Xenograft and Porcine-Derived Resorbable Membrane in Augmentation of Posterior Extraction Sockets with a Severe Wall Defect. A Radiographic/Tomographic Evaluation. J Oral Maxillofac Res. 2019 Mar 31;10(1):e3. doi: 10.5037/jomr.2019.10103. PMID: 31086644; PMCID: PMC6498814.
- Method of Preparing Porous Carbonate Apatite from Natural Bone. United States Patent US 8,980,328
- F Landi E., Celotti G., Logroscino G., Tampieri A. 2003. Carbonated Hydroxyapatite as Bone Substitute. Journal of the European Ceramic Society 23: 2931–2937.
- Spense G., Patel N., Brooks R., Rushton N. 2009. Carbonate Substituted Hydroxyapatite: Resorption by Osteoclasts Modifi es the Osteoblastic Response. Journal of Biomedical Materials Research Part A 217-224.
- Doi Y, Shibutani T, Moriwaki Y, Kajimoto T, Iwayama Y. Sintered carbonate apatites as bioresorbable bone substitutes. J Biomed Mater Res 1998;39:603–610
- Hasegawa M, Doi Y, Uchida A. Cell-mediated bioresorption of sintered carbonate apatite in rabbits. J Bone Joint Surg [Br] 2003;85:142–147.
- Spense G., Patel N., Brooks R., Rushton N. 2009. Carbonate Substituted Hydroxyapatite: Resorption by Osteoclasts Modifi es the Osteoblastic Response. Journal of Biomedical Materials Research Part A 217-224.
- Method of Preparing Porous Carbonate Apatite from Natural Bone. United States Patent US 8,980,328.
- Muzaffer A et al. ‘The Effect of Hyaluronic Acid-supplemented Bone Graft in Bone Healing: Experimental Study in Rabbits.’J Biomater Appl 2006 20:209
- Sasaki T, Watanabe C. ‘Stimulation of osteoinduction in bone wound healing by high-molecular hyaluronic acid.’ Bone. Vol. 16. No.1 January 1995:9-15
- Stiller M et al. ‘Performance of β-tricalcium phosphate granules and putty, bone grafting materials after bilateral sinus floor augmentation in humans.’ Biomaterials 2014;35(10):3154-3163.
- Mendes RM et al. ‘Sodium hyaluronate accelerates the healing process in tooth sockets of rat.’ Arch Oral Biol 2008; 53:1155–1162
- King, S.R., Hickerson, W.L. and Proctor, K.G. (1991) Benefi cial Actions of Exogenous Hyaluronic Acid on Wound Healing. Surgery, 109, 76-86.
- Asparuhova M, Kiryak D, Eliezer M, Mihov D, Sculean A. ‘Activity of two hyaluronan preparations on primary human oral fi broblasts’. J Periodontal Res 2018 Sep 27. Epub 2018 Sep 27
- Pirnazar P et al. ’Bacteriostatic effects of hyaluronic acid.’ Journal of Periodontology 1999;70:370-374
- Internal testing results, data on file.
- Internal testing results, data on file.
- Eliezer M, Sculean A, Miron RJ, et al. ‘Hyaluronic acid slows down collagen membrane degradation in uncontrolled diabetic rats.’ J Periodontal Res. 2019;00:1–9. https://doi.org/10.1111/jre.12665
- Brett D. A Review of Collagen and Collagen-based Wound Dressings. Wounds 2008;20(12).
- Data on file